In the fast-evolving world of biotechnology, precise and reliable analytical techniques are essential for research, development, and quality control. One powerful technique that has gained prominence across many scientific fields including biotech is X-ray Fluorescence (XRF). Whether you’re studying biomaterials, developing diagnostic devices, or ensuring the purity of pharmaceutical products, understanding XRF can significantly enhance your analytical toolkit.
What is XRF (X-ray Fluorescence)?
X-ray Fluorescence (XRF) is a non-destructive analytical method used to determine the elemental composition of materials by measuring the characteristic secondary X-rays emitted from a sample when it is excited by a primary X-ray source.
Unlike many analytical techniques that require complex sample preparation or destruction of the sample, XRF offers rapid, multi-element detection with minimal sample prep. It can detect elements from sodium (Na, atomic number 11) all the way up to uranium (U, atomic number 92), making it ideal for analyzing a wide array of biological, synthetic, and mineral samples relevant in biotechnology.
The Science Behind XRF: How Does It Work?
XRF’s principle is based on atomic physics and the unique way that atoms absorb and emit X-rays.
Step 1: Excitation by Primary X-rays
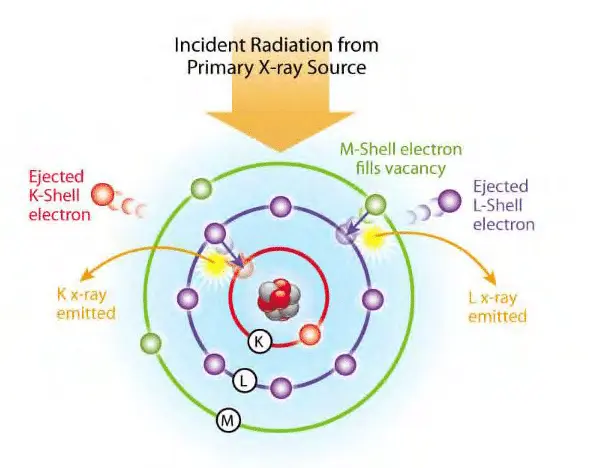
The XRF instrument emits primary X-rays, which strike the sample surface. This primary radiation has enough energy to excite atoms by ejecting electrons from their inner electron shells (usually the K or L shells).
Step 2: Electron Vacancy Creation
When an inner shell electron is ejected, the atom becomes unstable due to the vacancy created.
Step 3: Electron Transition and Fluorescence
To regain stability, an electron from a higher energy shell drops down to fill the vacancy. This transition releases energy in the form of a fluorescent (secondary) X-ray photon. The energy of this fluorescent X-ray is unique and characteristic for each element, much like a fingerprint.
Step 4: Detection of Fluorescent X-rays
The XRF detector captures these emitted X-rays and measures their energy and intensity.
Step 5: Element Identification and Quantification
By analyzing the energy spectrum, the instrument identifies which elements are present. The intensity of each characteristic peak correlates with the concentration of the corresponding element in the sample.
Types of XRF Technology
XRF instrumentation can be broadly classified into:
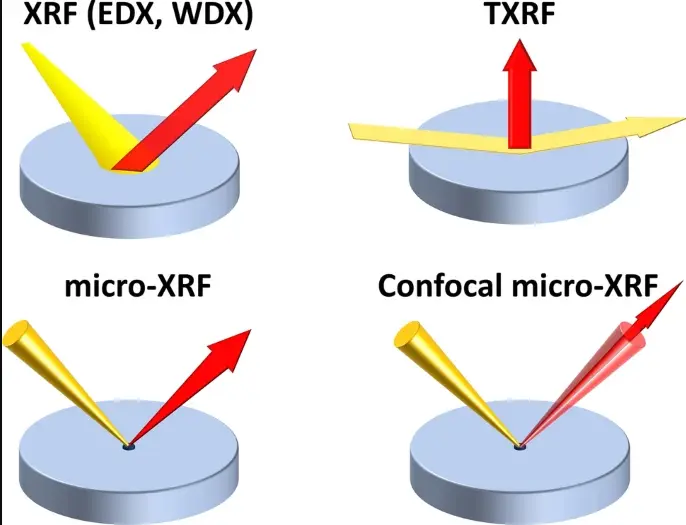
1. Energy Dispersive XRF (EDXRF)
The most common type, it uses a solid-state detector to measure the energy of the fluorescent X-rays directly, enabling simultaneous detection of multiple elements quickly.
2. Wavelength Dispersive XRF (WDXRF)
Utilizes diffraction crystals to separate X-rays by wavelength for highly precise and accurate elemental analysis, especially valuable when trace level detection or element overlap occurs.
3. Micro-XRF
Provides elemental mapping with micrometer spatial resolution, ideal for analyzing small biological samples, tissues, or biomaterials.
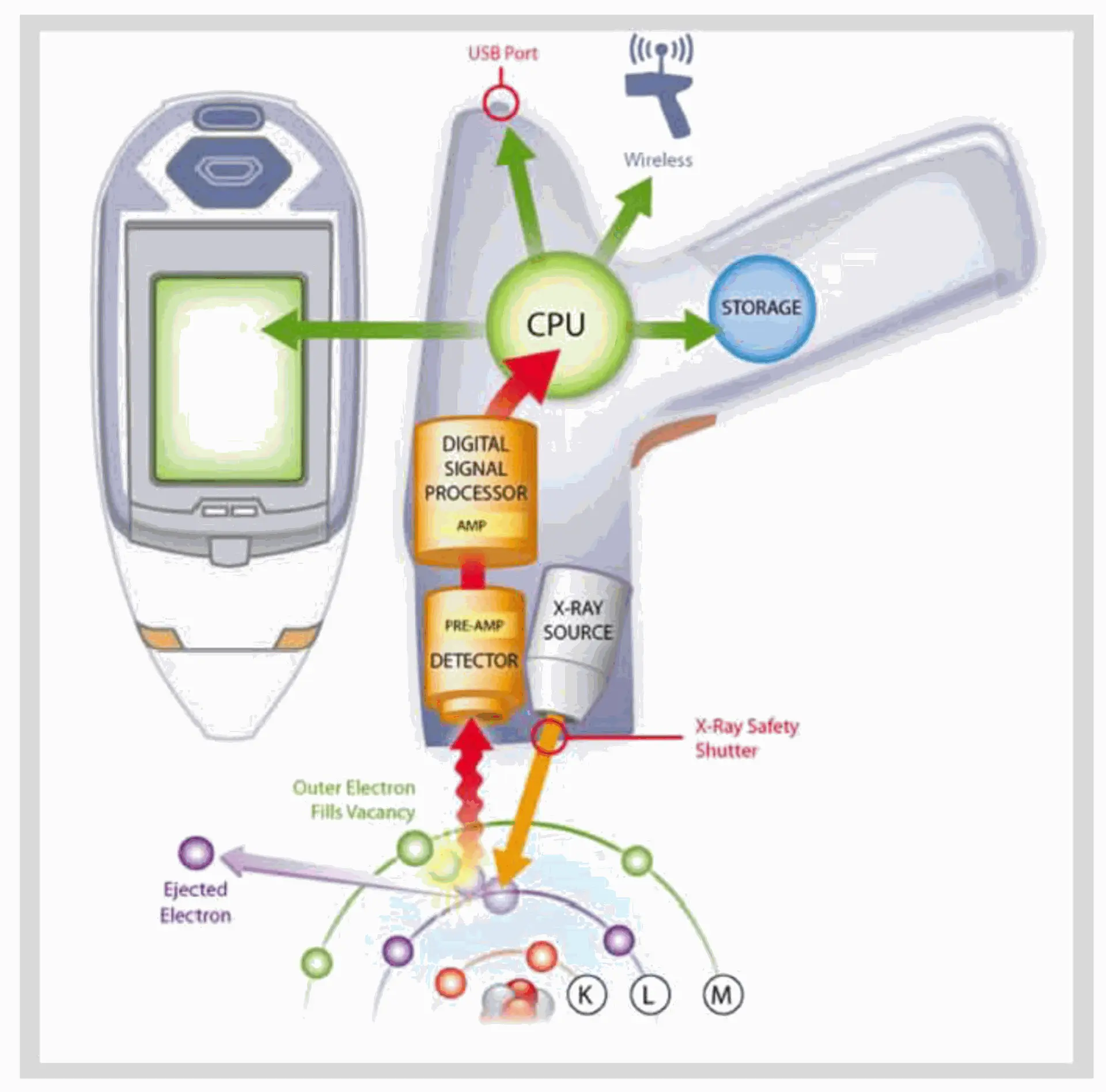
4. Portable/Handheld XRF
Compact instruments designed for in-field elemental analysis, valuable in environmental biotech and on-site quality control.
Applications of XRF in Biotechnology
Though traditionally associated with geology, mining, and materials science, XRF has several important roles in biotechnology:
1. Biomaterials Analysis
Characterizing the elemental composition of implants, scaffolds, or bioactive glasses used in tissue engineering to ensure biocompatibility and safety.
2. Trace Metal Analysis in Biological Samples
Monitoring essential or toxic trace elements in biological fluids, tissues, or environmental samples, supporting toxicology and pharmacokinetics studies.
3. Quality Control in Pharma and Biotech Manufacturing
Verifying the purity of raw materials and final products by detecting elemental contaminants or confirming formulation consistency.
4. Environmental Biotechnology
Analyzing soil and water samples for heavy metal contamination, crucial for bioremediation and ecological risk assessment.
5. Diagnostic Device Development
Material verification of biosensor components, ensuring proper elemental makeup for optimal performance.
6. Food Biotechnology
Detection of mineral nutrients and contaminants to guarantee food safety and nutritional content.
Advantages of XRF for Biotech Professionals
- Non-destructive: Samples remain intact, preserving precious biological or medical materials.
- Rapid and Multi-elemental: Simultaneously detects multiple elements in seconds.
- Minimal Sample Prep: Often requires little to no sample preparation.
- Wide Elemental Range: Detects elements from Na to U, useful for diverse samples.
- Portable Options: Enables field testing, environmental monitoring, and on-site QC.
- Quantitative and Qualitative: Provides both elemental identification and concentration data.
How XRF Fits Into Your Biotech Workflow
XRF complements other analytical techniques such as ICP-MS (Inductively Coupled Plasma Mass Spectrometry), AAS (Atomic Absorption Spectroscopy), and electron microscopy by providing fast elemental analysis with minimal sample destruction. It is especially useful for:
- Preliminary screening of samples for elemental content
- Quality control checks during manufacturing
- Environmental monitoring in bioprocessing sites
- Research into biomaterial composition and interactions
Final Thoughts
XRF (X-ray Fluorescence) is a versatile, fast, and non-destructive technique that holds immense value in biotechnology. From ensuring the elemental purity of biomaterials to monitoring trace metals in biological samples and environmental contexts, XRF can be a critical part of your analytical arsenal.
Last News :
Portable X-ray Fluorescence Analysis of Organic Amendments: A Review
X-Ray Fluorescence Spectrometry: Current Status and Prospects of Development