Immunology Protocols: Key Techniques for Advanced Immune Research
Antibody Cleanup: Ensuring Purity for Immunoassays
Antibody cleanup is essential for removing impurities and contaminants from antibody samples, ensuring that they function optimally in various immunoassays. This protocol is crucial for improving the sensitivity and specificity of experiments such as ELISA, Western blot, and immunohistochemistry, by using purified antibodies for accurate detection of antigens.
Multiple and Portable ELISA (M&P ELISA): Enhanced Immunoassay Efficiency
The Multiple and Portable ELISA (M&P ELISA) protocol offers a flexible approach to conducting enzyme-linked immunosorbent assays (ELISA) in various settings. This technique allows for simultaneous detection of multiple analytes in a compact format, making it a valuable tool in diagnostics, research, and point-of-care testing, particularly in resource-limited environments.
Immunoperoxidase Staining of Cultured Cells: Visualizing Protein Expression
Immunoperoxidase staining is a widely used method for detecting specific proteins in cultured cells. By utilizing an enzyme-linked antibody that reacts with a chromogenic substrate, this protocol provides a clear and visible signal, allowing researchers to study protein expression and localization in cellular models, often used in cancer and infectious disease research.
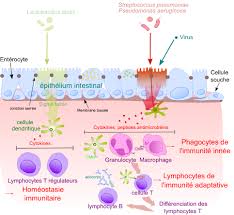
ELISPOT Protocol: Detecting Single-Cell Immune Responses
The ELISPOT (Enzyme-Linked ImmunoSpot) assay is a sensitive technique used to detect cytokine production at the single-cell level. This protocol is particularly useful for monitoring immune responses in individual cells, providing insights into the functionality of immune cells in studies of infections, vaccines, and immunotherapy.
ChIP Assay Protocols: Exploring Protein-DNA Interactions in Immunology
Chromatin immunoprecipitation (ChIP) assays are used to study protein-DNA interactions, especially transcription factors and histone modifications that regulate immune genes. This protocol is valuable for investigating how immune responses are regulated at the genetic level and for understanding epigenetic changes during immune cell differentiation.
Modified Immunohistochemical Protocol for Paraffin Embedded Sections: Preserving Tissue Integrity
Immunohistochemistry (IHC) on paraffin-embedded sections is a standard method for studying protein expression in tissue samples. This modified protocol enhances antigen retrieval and staining efficiency, making it ideal for archival samples in pathology, cancer research, and immune tissue studies.
Immunohistochemistry: Retrievals for Optimal Antigen Detection
Antigen retrieval is a critical step in immunohistochemistry that restores antigenicity in formalin-fixed, paraffin-embedded tissues. This protocol outlines various retrieval methods, such as heat-induced and enzymatic antigen retrieval, which are essential for improving the detection of immune markers in histological sections.
NIH Protocols for Immunohistochemistry: Standardized Procedures for Reproducibility
The National Institutes of Health (NIH) have established standardized protocols for immunohistochemistry (IHC), ensuring reproducibility and consistency in immunological research. This protocol is used across labs worldwide for studying immune markers, disease progression, and therapeutic responses in tissues.
B Cell and T Cell Isolation: Purifying Immune Cell Populations
This protocol outlines methods for isolating B cells and T cells from blood or tissues using magnetic beads or flow cytometry. Purified immune cells are critical for studying their roles in adaptive immunity, vaccine development, and immune disorders, allowing researchers to explore cell-specific functions.
Troubleshooting Guide for Immunohistochemistry: Ensuring Reliable Results
This guide provides solutions to common problems encountered during immunohistochemistry, such as poor staining, non-specific binding, or weak signal intensity. By following this protocol, researchers can troubleshoot and optimize their experiments, leading to more reliable and interpretable results in tissue-based studies.
Grocott’s Methenamine Silver (GMS) Stain: Detecting Fungal Infections
GMS staining is a histochemical method used to visualize fungi in tissue samples. This protocol is critical for diagnosing fungal infections in clinical and research settings, particularly in immunocompromised patients, by highlighting fungal cell walls in tissue sections.
Elegans Immunohistochemistry: Studying Immune Pathways in a Model Organism
The nematode Caenorhabditis elegans is an important model for studying innate immunity. This protocol provides methods for immunohistochemical staining of C. elegans, allowing researchers to explore immune signaling pathways, pathogen-host interactions, and the effects of mutations on immune responses.
Cytokine Assay Protocols: Measuring Immune Signaling Molecules
Cytokines play a pivotal role in immune regulation and inflammation. This protocol offers various methods for quantifying cytokines in biological samples, including ELISA, bead-based assays, and multiplex platforms, making it essential for studies of immune responses, inflammation, and therapeutic interventions.
Fluorescence In Situ Hybridization (FISH): Visualizing Genetic Information
FISH is a powerful technique used to detect specific DNA or RNA sequences within cells, combining immunology with genomics. This protocol is particularly useful for identifying genetic abnormalities, chromosomal rearrangements, and gene expression patterns in immune cells, aiding in the diagnosis of immune-related disorders.
Radioimmunoassay (RIA) Protocols: Detecting Hormones and Antigens with High Sensitivity
Radioimmunoassays (RIA) are highly sensitive techniques for measuring low concentrations of hormones, antigens, and antibodies in biological samples. This protocol is widely used in immunology and endocrinology to detect molecules such as cytokines, insulin, and viral antigens.
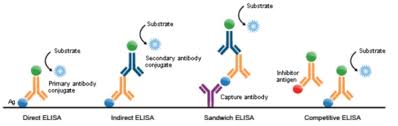
Competitive ELISA Protocols: Quantifying Antigens in Complex Samples
Competitive ELISA is a variant of ELISA that is used to measure the concentration of antigens in complex samples. This protocol is valuable for detecting low-abundance antigens in serum, plasma, or tissue extracts, providing insights into immune responses, infections, and autoimmune diseases.
Immunohistochemistry Stain for Frozen Tissue: Preserving Antigen Integrity
This protocol describes the use of immunohistochemistry on frozen tissue sections, which preserves antigen integrity better than paraffin-embedded samples. Frozen tissue IHC is commonly used in immune studies, especially when immediate fixation is required, such as in biopsies or rapidly changing immune environments.
These protocols provide essential tools for studying immune function, disease mechanisms, and therapeutic development in the field of immunology.